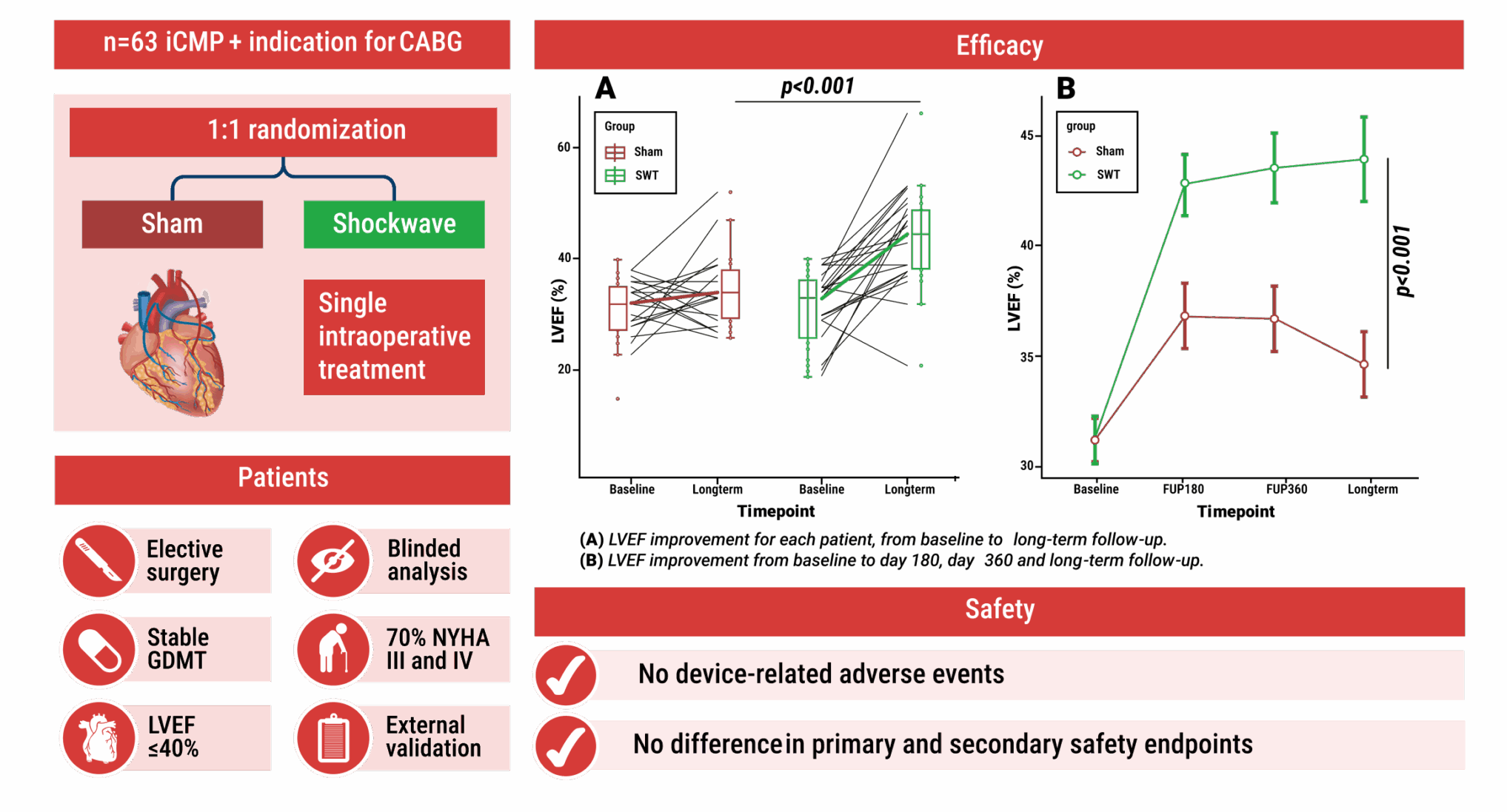
Chronic ischaemic heart failure remains a major challenge as revascularisation strategies relieve symptoms but often fail to restore left ventricular function. Cardiac shockwave therapy (SWT) is a novel therapeutic approach that aims to stimulate angiogenesis and myocardial repair in hibernating myocardium. To investigate this potential, the CAST-HF trial was conducted as a single-blind, parallel-group, sham-controlled study. Patients with left ventricular ejection fraction (LVEF) ≤40% undergoing coronary artery bypass grafting (CABG) were randomly assigned to receive intraoperative direct cardiac SWT or sham treatment in addition to surgery. The primary endpoint was the change in LVEF assessed by cardiac magnetic resonance imaging (CMR) from baseline to 12 months, while functional capacity and quality of life served as secondary endpoints.

63

2021
up to 4 years